Topic 5 – 3D Vascularized Strategies to Restore Healthy or Tumour organoids Microenvironment
POINTED (Patient’s Organoids for Innovative ThErapeutic Device)
Technological and medical advances in recent decades have enabled our society to provide better care for sick people with an improvement in the quality of life and an increase in the survival rate in the face of infectious, cardiovascular and cancer diseases. Despite this, current treatments are not effective for everyone, some are accompanied by serious side effects, or still others see their effectiveness decrease with the increase in resistance mechanisms, for example. These treatments are therefore unsuitable, with excessive toxicity and ineffectiveness.
At present, about 95% of new therapeutic molecules fail before the end of the clinical process, leading to a considerable waste of time and money. On average, 7.3 years and $ 648 million are required to develop a new therapeutic molecule. The scientific community agrees that this failure rate is not related to the active molecules themselves, but to the experimental models used to select them. It is from this perspective that in vitro drug testing has evolved into 3D culture, called microtissus, spheroids or organoids, much more selective than 2D culture. These tests allow the use of human cells but do not have vascularity, and thus do not represent the systemic distribution of the drug, making these tests poorly predictive and irrelevant. In contrast, preclinical screening tests are certainly systemic, but unfortunately not human. The missing link between these two models leads to a bias in the selection of therapeutic molecules, creating this considerable failure rate.
Beyond the flaws that exist in the drug development process, once on the market, drugs do not have an absolute success rate either. In the context of cancer, for example, it was estimated in the United States in 2019 that there were only 33% of survivors 5 years after diagnosis. This means that in about 70% of cases, the treatments are ineffective. This is the case for many other pathologies, such as the increase in the number of cases of resistance to tuberculosis treatment (10% since 2014); diabetes and its consequences such as myocardial infarction (1,000 deaths out of 10,000 hospitalized diabetics), nephropathies (3,000 diabetics per year); or hypertension, which is the leading cause of cardiovascular complications, such as stroke, and in which 30% of patients are resistant to treatment.
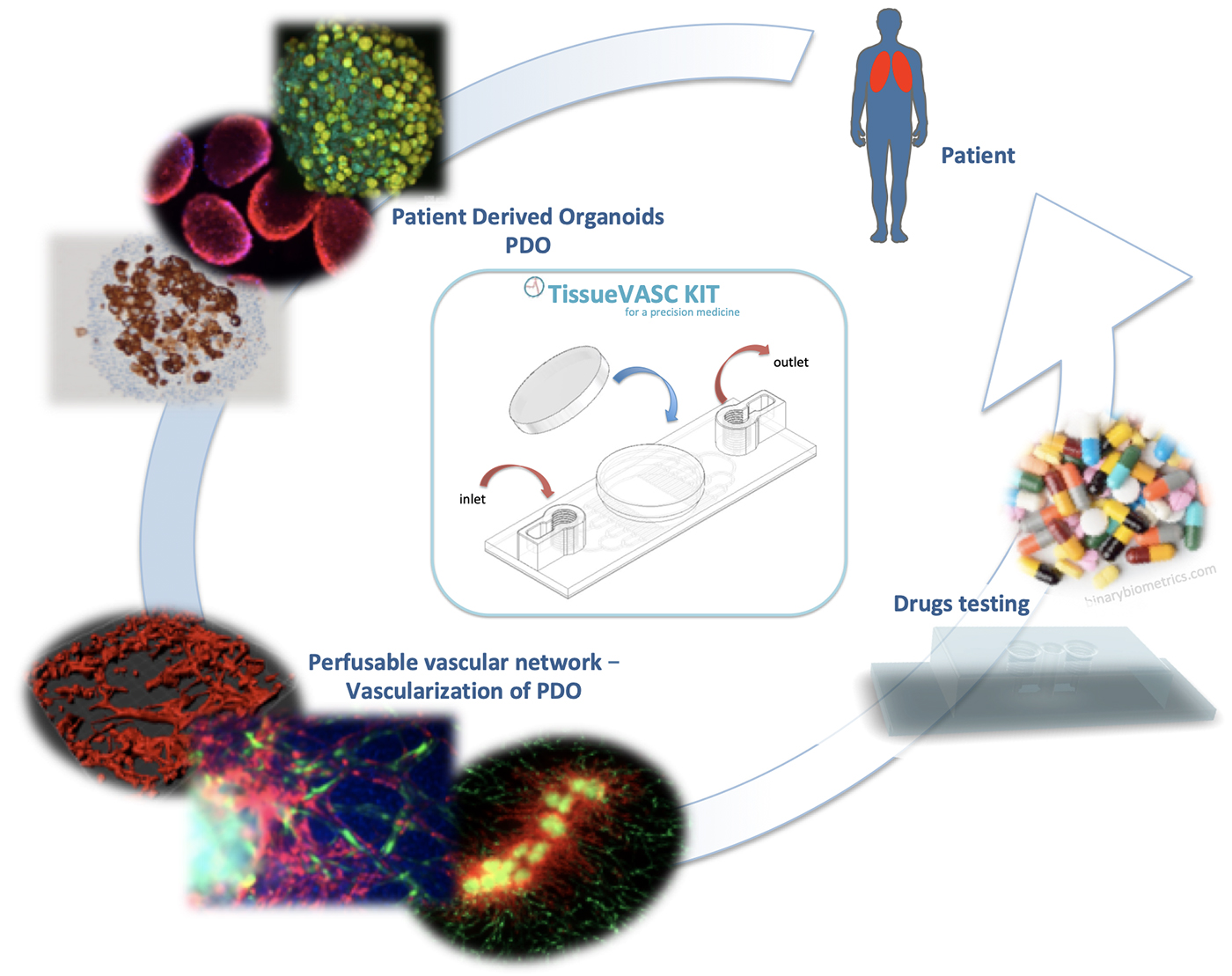
It is important to underline two limitations that are still lacking i) the lack of vascularization in in vitro tests during drug development, a determining parameter of drug distribution; ii) lack of personal adjustment when treating a patient.
A new paradigm in material synthesis is emerging, in which living cells are incorporated into non-living matter. This combination of synthetic biology tools in materials research promises to deliver a new generation of high-performance and smart material systems. Engineered Living Materials (ELMs) open new opportunities for more sustainable material production and advanced property combinations like integrated multifunctionality and adaptability. Our project will bridge materials science with the synthetic biology, biotechnology, and biophysics communities to address the compositional and technical challenges for a functional association of non-living matter and living components, and the experimental and computational solutions to realize them. The engineered living material would push the boundaries and frontiers of synthetic biology, materials engineering, nanotechnology, biomaterials and artificial intelligence.
Living materials challenge is to recapitulate all characteristics of biological systems: self-replication, self-regulation, self-healing, environmental responsiveness and self-sustainability; Engineered Living Materials are defined as engineered materials composed of living cells that form or assemble the material itself or modulate the functional performance of the material in some manner.
Objectives:
Design and optimization of an innovative medical device to improve therapies based on well-organized and vascularized organoids (ELMs) derived from patient tissue for precision medicine: TissueVASC kit ready-to-use for personalized medicine.
We focus on different types of cancers such as lung cancer, glioblastoma or osteosarcoma as models for developing this medical device. However, this medical device can be transposed to any type of pathology, since the objective is to reconstitute any tissue and to vascularize it in a device allowing its perfusion.
Team members of Topic 5
| Name |
Position |
Phone |
e-mail |
Nadia BENKIRANE-JESSEL |
INSERM Research Director (DR1) |
+33 (0)3 68 85 33 76 |
nadia.jessel@inserm.fr |
Julien DEMISELLE |
Clinician PhD student |
+33 (0)3 69 55 12 12 |
julien.demiselle@chru-strasbourg.fr |
Pierre-Emmanuel FALCOZ |
PU-PH |
+33 (0)3 69 55 07 96 |
pierre-emmanuel.falcoz@chru-strasbourg.fr |
Ezeddine HARMOUCH |
Post-doc |
+33 (0)3 68 85 38 68 |
ezeddine.harmouch@etu.unistra.fr |
Jacky Guoqiang HUA |
Associate Professor |
+33 (0)3 68 85 33 76 |
g.hua@unistra.fr |
Ysia IDOUX-GILLET |
Research Engineer |
+33 (0)3 68 85 38 68 |
yidouxgillet@unistra.fr |
Hélène LE |
Ph.D. Student |
+33 (0)3 68 85 33 76 |
le@transgene.fr |
Véronique LINDNER |
PH |
+33 (0)3 69 55 07 49 / +33 (0)3 88 12 79 96 |
veronique.lindner@chru-strasbourg.fr |
Thierry MASSFELDER |
INSERM Research Director |
+33 (0)3 68 85 33 76 |
massfeld@unistra.fr |
Guy FUHRMANN |
CNRS Researcher |
guy.fuhrmann@unistra.fr |
|
Charlotte PONTE |
Clinician PhD student |
charlotte.ponte@chru-strasbourg.fr |
|
Anas NOUNSI |
Ph.D. Student |
+33 (0)3 68 85 33 78 |
anasse.nounsi@etu.unistra.fr |
Anne OLLAND |
PU-PH |
+33 (0)3 69 55 11 31 |
anne.olland@chru-strasbourg.fr |
Joseph SEITLINGER |
Clinician PhD student |
+33 (0)3 83 15 42 84 |
joseph.seitlinger@etu.unistra.fr |
Geneviève UBEAUD-SEQUIER |
PU-PH |
+33 (0)3 68 85 42 11 |
ubeaud@unistra.fr |
Prizes and awards:
- Laureate at the International Organoid Contest (INOContest) 2019
- Laureate at the Fond’Action Alsace Awards 2021




