Topic 1 – Advanced Therapeutics for Bone and Cartilage Regeneration
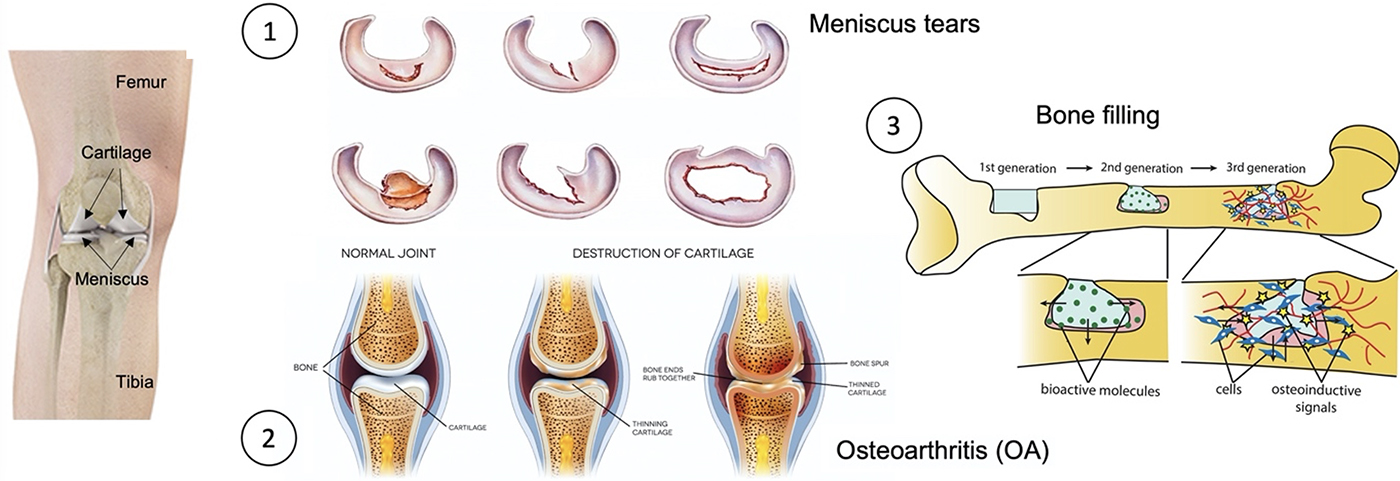
Presentation
Regenerative medicine has burgeoned due to recent advances in stem cells and biomaterials to meet the goal of cell and organ replacement to restore function resulting from degeneration, aging, trauma, or other disease processes. The objective of our topic is to provide better clinical treatment to patients suffering from bone and cartilage diseases, using our knowledges and cutting-edge biotechnologies.
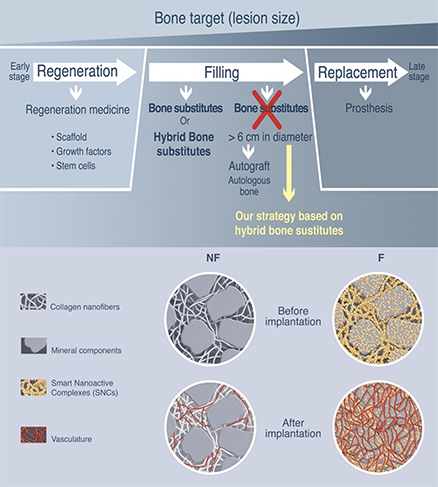
Bone defects can be caused by different origins, such as trauma, tumor or infection, and regeneration, filling and replacement are different strategies for bone repair according to the size of lesion. To avoid the total replacement of bone by prothesis, we have developed two different strategies according to patient’s need to either regenerate or fill bone lesions.
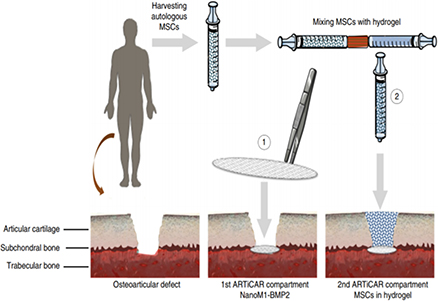
A therapeutic wound dressing technology to prevent early osteoarthritis following traumatic knee injuries
Osteoarthritis (OA) is one of the most widespread chronic diseases in adults. By 2030, about 20% of adult people will be affected by OA in industrialized countries. About 6 million people in France suffer from OA. Knee injury is one of the strongest risk factors for OA. Today, the main techniques involved in OA lesions repair present important constraints with limited efficiency or side effects, such as fibrocartilage formation, hypertrophic or hypotrophic cellular content with subsequent degeneration, and inadequate linking to the host bone/cartilage. The need for more efficient therapies is tremendous. In this context, our objective is to regenerate the cartilage in a more stable way through the regeneration of the subchondral bone, acting as glue between bone and cartilage.
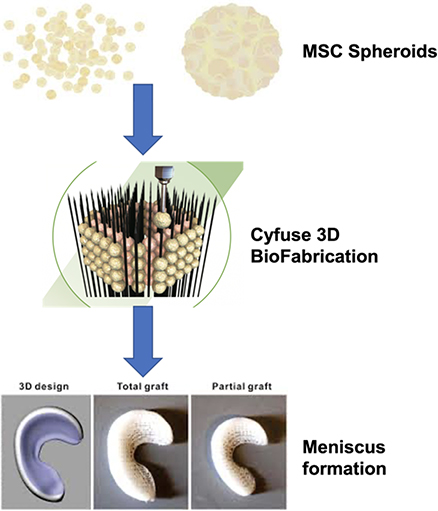
Design and Optimization of a Combined Therapeutic Implant based on Stem Cells Spheroids for Meniscal Repair
The menisci are two crescent-shaped fibrocartilaginous structures in the knee, playing an important protective role for the knee joint through shock absorption, load distribution and joint lubrication due to their deformable shape and mobile nature. Unfortunately, the meniscal injuries are quite common with a 61 per 100,000 incidence rate of meniscal tears in the general population. Owing to the hypovascular and hypocellular properties, meniscus tears could not heal spontaneously, thus, leading to knee OA. We propose an innovative 3D bioengineering Cyfuse S-PIKE technology combined with mesenchymal stem cells (MSCs) to regenerate and replace the meniscus with better safety and accuracy.
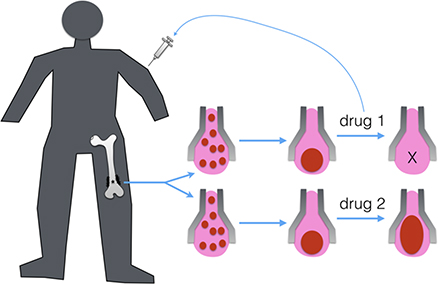
Patient-derived vascularized minitissue based microfluidic drug screening device
Osteosarcoma is the most common primary bone malignancy presenting typically during childhood and adolescence. However, few improvements of the survival outcomes for osteosarcoma patients have been achieved since the last three decades. Despite the rarity of the diagnosis, the complexity of tumour microenvironment and the genetic heterogeneity remain the major obstacles to understanding the mechanisms involved in the tumour progression and metastasis, and to screening the pharmacologically active molecules for better drugs. In this context, our objective is to develop an innovative drug-screening device which combines the patient-derived vascularized tumour or healthy minitissue with a perfusable microfluidic network to test therapeutic molecules and provide a rapid and personalized clinical treatment to the patient.
Scientific expertise
- Bone filling and regeneration
- Electrospinning of thin and thick biomaterial scaffolds
- Hybrid bone substitutes
- Nanofunctionalization
- Stem cell therapies, therapeutic scaffolds, combined Advanced Therapy Medicinal Product (cATMPs)
- In vitro and In vivo assays
- Preclinical and Clinical trials
- Cartilage regeneration
- Electrospinning of nanofibrous membrane
- Hydrogel formulation
- Nanofunctionalization
- 3D Organoid Stem cell therapies
- In vitro and In vivo assays
- Preclinical and Clinical trials
- Meniscus regeneration and replacement
- Next generation 3D biofabrication technology without ink (Cyfuse S-PIKE)
- Mesoscale tissue biofabrication
- 3D well-organized Organoid Stem cell therapies
- In vitro and In vivo assays
- Preclinical and Clinical trials
- Personalized Osteosarcoma drug screening
- Next generation 3D biofabrication technology without ink (Cyfuse S-PIKE)
- Mesoscale tissue biofabrication
- Vascularized Hydrogel formulation
- In vitro and In vivo assays
- Microfluidic Chip device
Team members of Topic 1
| Name |
Position |
Phone |
e-mail |
Nadia BENKIRANE-JESSEL |
INSERM Research Director (DR1) |
nadia.jessel@inserm.fr |
|
Jacky Guoqiang HUA |
Associate Professor |
g.hua@unistra.fr |
|
Florence FIORETTI |
Associate Professor / Oral surgeon |
fiorettioce@gmail.com |
|
Fabien BORNERT |
Associate Professor / Oral surgeon |
bornertfabien@gmail.com |
|
Xavier van BELLINGHEN |
Associate Professor / Oral surgeon |
vanbellinghen@unistra.fr |
|
Catherine-Isabelle GROS |
Associate Professor / Oral surgeon |
gros.catherine@unistra.fr |
|
Damien OFFNER |
Associate Professor / Oral surgeon |
damien.offner@hotmail.fr |
|
Gabriel Fernandez DE GRADO |
Associate Professor / Oral surgeon |
fernandezdegrado@unistra.fr |
|
Anne-Marie MUSSET |
Associate Professor / Oral surgeon |
annemarie.musset@chru-strasbourg.fr |
|
Rana SMAIDA |
Ph.D. Student |
ranasmaida@hotmail.com |
|
Anas NOUNSSI |
Ph.D. Student |
anas-2020@hotmail.fr |
|
Moustafa NAJA |
Ph.D. Student |
moustafanaja@gmail.com |
International Patents
- CNRS/INSERM: WO2006/079928: PCT Europe and US 2007
- INSERM/CNRS: JWO2007132099-A2; FR2901143-A1; US2009239302-A1
- Biomaterials comprising a scaffold containing a mineral compound, and uses thereof as bone substitutes – N. Ref. BET 18P0150 (STL-EC), EP 19305180.2
- A compound compressing alpha-MSH for use in Endodontic Regeneration. INSERM/ University of Strasbourg, 2010: PCT/IB2010/003458
- Nanoreservoirs technology for use in bone and /or cartilage regeneration. INSERM/ University of Strasbourg, 2011 / BET 10P2884 Europe n°11305182.5; EP N° 11305182.5 du 22/02/2011, International: n° PCT/EP2012/052976; Japan, 26/08/2016, n°5990720
- Three-dimensional scaffold functionalized with MT for tissue regeneration: EB3008-AF. BET13P2142, 2013
- TumVascAssay: INSERM/ University of Strasbourg Patent PR1691, application filed in December 2014
- Biomaterials comprising a scaffold containing a mineral compound, and uses thereof as bone substitutes: PCT/EP2020/053761. US application or PCT International Application filed on 13 February 2021.
Lamina Therapeutics

Based on more than 10-year’s research experience on tissue engineering, we have developed several validated innovative strategies and strong pipeline for bone and cartilage regeneration. Based on the solid preclinical data according to the international regulatory guidelines, a Start-up called Lamina Therapeutics has been set up in 2020. The phase I clinical trial for the product “Lamina One” will be started in 2022 in several countries in Europe.
Most Relevant Publications of the topic 1
- Proc Natl Acad Sci USA. 1997, 94 :12545-12550 (IF-12.291)
- Adv. Mater. 2004, 15 :692-695 (IF-21.950)
- Adv. Materials, 2004, 16, 1507-1511 (IF-21.950)
- Adv. Materials, 2004, 14, 963-969 (IF-21.950)
- Adv. Funct. Mater. 2004, 14 :174-182 (IF-13.325)
- Adv. Funct. Mater. 2005, 15 :648-654 (IF-13.325)
- Proc Natl Acad Sci USA. 2006, 103 :8618-8621 (IF-12.291)
- Biomaterials. 2006, 27 :1771-1777 (IF-8.312)
- Adv Mater 2007, 19: 693-697 (IF-21.950)
- Small 2007, 3: 1577-1583 (IF-7.514)
- Nano Letters 2008, 8: 2432-2436 (IF-12.94)
- Biomaterials 2009, 30: 6367-6373 (IF-8.806)
- Adv Mater 2009, 2: 650-655 (IF-21.950)
- Biomaterials 2010, 31: 1699-1706 (IF- 8.806)
- Proc Natl Acad Sci, 2010, 107, 3406-3411 (IF-12.291)
- ACS Nano 2010, 4, 3277-87 (IF-13.709)
- ACS Nano 2011, 5: 4790-4799 (IF-13.709)
- ACS Nano, 2012, 6, 483-490 (IF-13.709)
- Nanomedicine (Lond), 2015 10, 2833-2845 (IF-6.692)
- Proc Natl Acad Sci USA, 2016 Feb 2;113(5): E626-34 (IF-12.291)
- Proc Natl Acad Sci USA, 2016 Feb 2;113(5): E635-43 (IF-12.291)
- Trends in Biotechnology, 2017, 35, 8–11 (IF-13.578)
- Nature Communications, 2019, 10, Article number: 2156 (IF-14.919)
- Proc Natl Acad Sci USA, 2019 Jul 9; 116(28): 14191-14199 (IF-12.291)
- Proc Natl Acad Sci USA, 2019 Jul 9;116(28): 14200-14209 (IF-12.291)
- Molecules. 2019; 24(16) (IF-4.411)
- Nanomedicine. 2020 Oct; 29:102253 (IF-5,307)
- Biomedicines. 2021, 9(8):952 (IF-6,081)
The complete publication list is available at https://www.regmed.fr/publications/


